Successful Transcatheter Aortic Valve Replacement After Iatrogenic Aortic Cusp Dissection After Treatment of Complex, Unprotected Left Main Bifurcation Coronary Artery Disease
Injoon Lee1*, Matthew P. Cauchi2, Jason Foerst1-3, Gary Swank1,2
1Section of Cardiology, Virginia-Tech Carilion School of Medicine, Roanoke, Virginia, USA
2Interventional Cardiology, Virginia-Tech Carilion School of Medicine, Roanoke, Virginia, USA
3Structural Cardiology, Virginia-Tech Carilion School of Medicine, Roanoke, Virginia, USA
Abstract
Currently no literature exists regarding the safety and feasibility of transcatheter aortic valve replacement (TAVR) after aortic cusp dissection. Aortic dissection is a rare, albeit potentially fatal complication related to percutaneous coronary intervention, especially when dealing with heavily calcified coronary disease involving the left main. Rapid stratification of patients in need of early invasive repair versus conservative observation is crucial to patient morbidity and mortality. We present a case of an elderly female with severe aortic stenosis who underwent TAVR after complex intervention of heavily calcified multi-vessel coronary artery disease involving the left main and bifurcation with high-risk Impella protected rotational atherectomy and culotte stenting complicated by left coronary cusp dissection and was managed conservatively without long-term sequelae. This case highlights the achievability of TAVR after aortic cusp dissection that was treated with conservative methods.
Case Presentation
An 84-year-old female with a pertinent history of Guillan-Barré Syndrome (GBS), severe symptomatic aortic stenosis (AS) and coronary artery disease with remote percutaneous intervention (PCI) to the proximal left anterior descending (LAD) and circumflex-marginal branch, presented to our facility for an elective coronary angiography in response to an abnormal stress test completed as an outpatient. The patient had been experiencing symptoms of atypical chest pain and progressive, functionally limiting dyspnea on exertion over a period of two months. Given advanced age and debility due to GBS, the patient underwent a pharmacologic nuclear myocardial perfusion scan which demonstrated a small sized, moderate intensity defect with partial reversibility in the anteroseptal wall. Cardiac catheterization demonstrated complex, eccentric and heavily calcified left main disease compromising the ostium of the LAD and left circumflex arteries, along with tandem severe lesions in the proximal and distal right coronary artery (RCA) (Figure 1. Panel A and B).
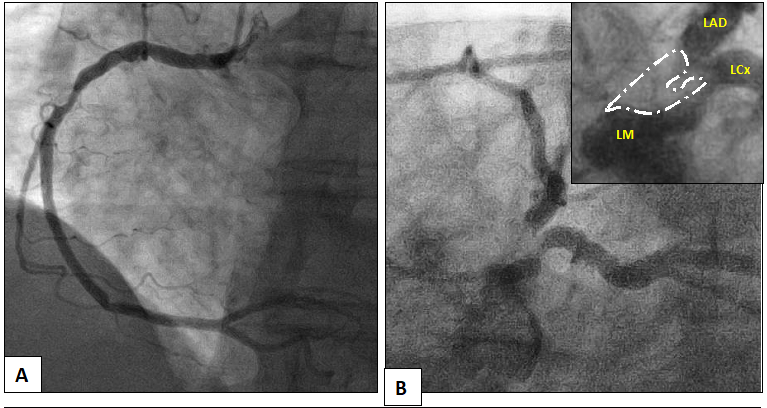
Figure 1: Initial diagnostic cardiac catheterization demonstrating severe disease in the right coronary artery (Panel A) and complex left main disease (Panel B). Inset shows complexity of left main disease (white = calcification).
Unfortunately, three months later, the patient presented to the Emergency Department with severe, typical chest pain radiating to her left arm and neck that resolved with sublingual nitroglycerin. Her EKG was negative for evidence of ischemia, but serum Troponin I levels were found to be mildly elevated (0.39 ng/mL; normal < 0.30 ng/mL) prompting admission to the inpatient Cardiology service. Given her presentation, the patient and family wished to pursue more aggressive percutaneous revascularization options and the decision was made to attempt high risk, Impella-protected left main rotational atherectomy and multi-vessel revascularization. An echocardiogram performed pre-procedure demonstrated an ejection fraction of 50-55%, severe aortic stenosis with mean gradient of 37 mmHg, peak gradient of 50 mmHg and an aortic valve area of 0.84 cm2.
Given the complexity of planned intervention and need for mechanical support due to unprotected left main disease, bilateral femoral artery access was obtained. The right coronary artery was engaged with a 6-French JR4 guide and wired without difficulty. The proximal and distal lesions were directly stented using 3.5 x 18 mm and 3.5 x 15 mm drug-eluting stents, respectively, and post-dilated with non-compliant balloons with excellent angiographic result (Figure 2). Right femoral artery angiography demonstrated suitable anatomy for mechanical support and the 5-French sheath was exchanged over a stiff wire for a 14-French sheath, though which the Impella CP was advanced into the left ventricle and position optimized (Level P9, Flow 3.3 L/min). After multiple attempts to wire through the left main into the LAD, an angled tip microcatheter was used to direct the wire down the LAD. During this process, the ostium of the left circumflex was dissected (Figure 3) but exhibited no evidence of compromised flow, so rotational atherecomy of the left main and ostial LAD using a 1.5 mm and 1.75 mm burr was completed without difficulty.
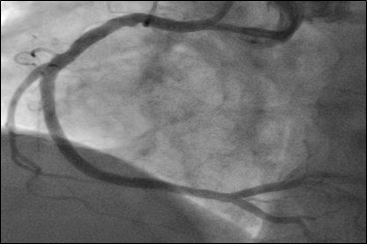
Figure 2: Angiography after uncomplicated PCI to the proximal and distal right coronary artery.
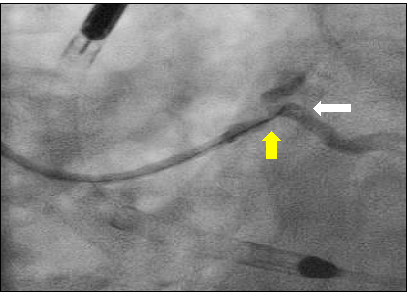
Figure 3: Angled tip microcatheter (yellow arrow) facilitating wiring of the LAD with wire induced dissection at the ostium of the left circumflex (white arrow).
After atherectomy, a culotte stent technique was performed utilizing 3.5 x 15 mm and 3.5 x 12 mm drug-eluting stents in LAD and left circumflex, respectively, extending back to the ostium of the left main and finished with a final kissing balloon technique. Final angiography demonstrated excellent expansion of the stents (Figure 4), but dye staining was noted in the left coronary cusp superior to left main indicative of a dissection (Figure 5).
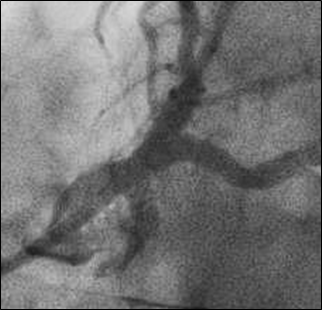
Figure 4: Angiography after culotte stenting and kissing balloon technique of left main bifurcation.
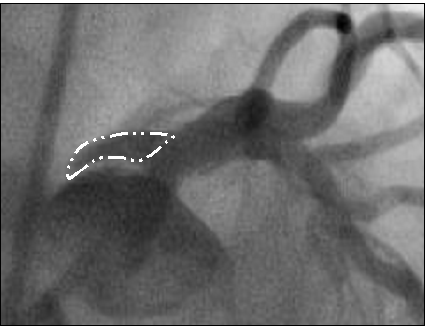
Figure 5: Left coronary cusp dissection (white outline).
The Impella was successfully weaned and removed in the catheterization lab prior to the patient returning to the floor. Post-procedure echocardiogram demonstated an ejection fraction of 60-65% but no gross evidence of aortic dissection. The patient remained asymptomatic and hemodynamically stable, so a conservative management was elected with close observation in the Cardiac Care Unit. Two days post-procedure, a CTA was completed demonstrating no further extension of the dissection (Figure 6. Panel A).
The patient was discharged home and underwent repeat imaging at one month, demonstrating complete resolution of the dissection (Figure 6. Panel B). Shortly thereafter, she was was readmitted for elective transcatheter aortic valve replacement with 26 mm Edwards Sapien 3 Valve, during which there was no evidence of coronary cusp dissection on root angiography at time of valve deployment (Figure 7. Panel A and B). Intra-op echo showed mean gradient of 6 mmHg and peak gradient of 15 mmHg. The patient’s post-procedure course was uncomplicated and subsequently discharged home the next day and has had no further complications in follow up.
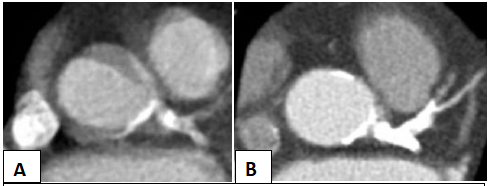
Figure 6: CT angiography two days post-catheterization (Panel A) and one month after discharge (Panel B).
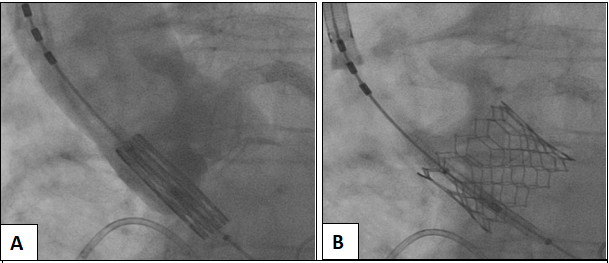
Figure 7: Aortography prior to balloon expandable 26 mm Edwards Sapien 3 TAVR valve deployment with no evidence of residual coronary cusp dissection (Panel A) and successful valve deployment (Panel B).
Discussion
Advancements in percutaneous techniques and equipment have provided many patients with significant co-morbidities considered inoperable or high risk for surgical procedures with minimally invasive options for the management of complex, high risk coronary artery and valve disease1. However, it comes at a price where the complication risk is much higher for these patients2.
Iatrogenic aortic dissection at the time of PCI is an uncommon complication, occurring at an overall frequency ranging from 0.03% to 1.9% and is typically due to trauma from guide catheters, high pressure balloon inflations, atherectomy, or hydraulic force of contrast injections2-5. For our patient, the combination of ostial calcification, atherectomy and aggressive kissing balloon post-dilatation were likely the main contributors to the development of the dissection. While the overall mortality for this complication is reported to be between 50-80%, those presenting with acute coronary syndrome have the highest mortality possibly related to aggressive antithrombotic agents and anticoagulation, along with a profound pro-inflammatory state6,7. Much like other types of aortic dissection, mortality depends on the rate of expansion and extent of dissection, which is most reliably evaluated and followed with serial CT or transesophageal echocardiography2. Unfortunately, there are no established guidelines in the management of iatrogenic aortic dissection, primarily due to the low incidence and individualized, multi-disciplinary approach necessary to ensure favorable outcomes. A more conservative approach involves strict control blood pressure to limit vascular trauma, serial imaging and close follow up, while more aggressive strategies include both percutaneous and open surgical repairs. Typically, more heroic procedures are necessary if the patient demonstrates hemodynamic instability, extensive propagation of the dissection, presence of a pericardial effusion, or aortic insufficiency. While percutaneous options are limited, some studies have advocated for placement of covered stents at the site of initial vascular trauma to thrombose the false lumen and limit the dissection plane7,8. Our patient remained hemodynamically stable evidence of dissection propagation, thus, we elected to treat her conservatively and was able to safely perform TAVR about one month later after demonstrating of healing.
To date, the feasibility of TAVR after aortic dissection has not been well described. Known aortic dissection complicates TAVR since one of the major predisposing factors for aortic wall complications include weakened arterial wall strength secondary to previous trauma. Thus, patients with previous aortic wall injuries are at highest risk for further dissection or perforation. However, due to the lack of data and literature, there isn’t a clear guideline in terms of how long to wait before further intervention, what factors are absolute contraindications versus relative contraindications before proceeding to TAVR after aortic cusp dissection. Patients with proximal coronary artery lesions and severe aortic stenosis are treated with staged PCI before TAVR or concomitant. However, when complications occur, it hinders the staged procedure, limiting further intervention at worst and delaying the procedure at best. Thus One of the advancements that is the used in high risk cases include mechanical circulatory support devices, mainly the Impella (Abiomed, Danvers, MA, USA), which has multiple indications including cardiogenic shock secondary to acute myocardial infarction (AMI), acute coronary syndrome (ACS) and high risk percutaneous coronary intervention (PCI)9,10. While the incidence of cardiogenic shock in respect to unprotected left main intervention remains low at 0.6-0.9%, there is a very mortality rate, in some studies as high as 80%, in the event of complications9,10. The Impella device has been demonstrated to lessen left ventricular workload and end diastolic pressure, allowing for maintenance of systemic and coronary perfusion despite profound cardiac dysfunction, ultimately improving in-hospital and 30-day mortality11. Furthermore, studies have also demonstrated that that use of the Impella device during high risk PCI reduced the risk of AKI, a major complication of cardiac catheterization with high morbidity and mortality12,13. Given our patient’s complex and heavily calcified left main disease involving the distal bifurcation and concomitant severe aortic valve disease, owing to a lack of cardiac reserve, performing this procedure without hemodynamic support would not be recommended given potential for minor complications having catastrophic results.
Our case demonstrates a successful case of TAVR after the upfront use of the Impella device in high risk unprotected left main interventions, as well as a rare and potentially disastrous complication of iatrogenic aortic cusp dissection managed conservatively with excellent results. Finally, patients with severe aortic stenosis also have similar risk factors for coronary artery disease, approaching concomitance as high as 70%, requiring multidisciplinary heart team approach in treating this complex patient population14.
Declaration
Ethics approval and consent to participate: The manuscript strictly adheres to the ethics committee guidelines.
Findings: There was no specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
All authors contributed and oversaw the case as well as the article presentation.
References
- Scheidt S, Wilner G, Mueller H, et al. Intra-aortic balloon counter-pulsation in cardiogenic shock. Report of a cooperative clinical trial. N Engl J Med. 1973; 288(19): 979-984.
- Boukhris M, Tomasello SD, Marza F, et al. Iatrogenic aortic dissection complicating percutaneous coronary intervention for chronic total occlusion. Can J Cardiol. 2015; 31: 320-7.
- Perez-Castellano N, Garcia-Fernandez MA, Garcia EJ, et al. Dissection of the aortic sinus of Valsava complicating coronary catheterization cause, mechanism evolution and management. Cathet Cardiovasc Diagn. 1998; 43: 273-9.
- El Sabbagh A, Patel VG, Jeroudi OM, et al. Angiographic success and procedural complications in patients undergoing retrograde percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 3282 patients from 26 studies. Int J Cardiol. 2014; 174: 243-8.
- Abdou SM, Wu CJ. Treatment of aortocoronary dissection complicating anomalous origin right coronary artery and chronic total intervention with intravascular ultrasound guided stenting. Catheter Cardiovasc Interv. 2011; 78: 914-9.
- Dunning DW, Kahn JK, Hawkins ET, et al. Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv. 2000; 51: 387-93.
- Lau CSM, McRoy GJ, Mahendraraj K, et al. Aortic Dissection after Percutaneous Coronary Intervention for Acute Coronary Syndrome An Outcomes-Based Study from the Nationwide Inpatient Sample Database. International Journal of Clinical Medicine. 2017; 8: 21-33.
- Li JC, Guan XI, Gong M, et al. Iatrogenic aortic dissection during percutaneous coronary intervention: A case report and review of the literature. J Int Med Res. 2018 Jan; 46(1): 526–532.
- Meraj PM, Doshi R, Schreiber T, et al. Impella 2.5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J Interv Cardiol 2017; 30: 256-263.
- Patel N, De Maria GL, Kassimis G, et al. Outcomes after emergency percutaneous coronary intervention in patients with unprotected left main stem occlusion: the BCIS national audit of percutaneous coronary intervention 6-year experience. JACC Cardiovasc Interv. 2014; 7: 969-80.
- Flaherty MP, Khan AR, O’Neil WW. Early Initiation of Impella in Acute Myocardial Infarction Complicated by Cardiogenic Shock Improves Survival: A Meta-Analysis. JACC Cardiovasc Interv. 2017; 10: 1805-1806.
- Flaherty MP, Pant S, Patel SV, et al. Hemodynamic Support With a Microaxial Percutaneous Left Ventricular Assist Device Impella Protects Against Acute Kidney Injury in Patients Undergoing High-Risk Percutaneous Coronary Intervention. Circ Res. 2017; 120: 692-700.
- Chakravarty T, Sharma R, Abramowitz Y, et al. Outcomes in Patients With Transcatheter Aortic Valve Replacement and Left Main Stenting. J AM Coll Cardiol. 2016; 67: 951-60.
- Hamm CW, Möllmann H, Holzhey D, et al. The German Aortic Valve Registry GARY in-hospital outcome. Eur Heart J. 2014; 35(24): 1588-98.
