Clinical Characteristics of Cardiac and Pericardial Metastasis in Small Cell Lung Cancer with Arrhythmia
Yuling He1, Jingjing Wang1, Lingdong Kong1, Bo Jia1, Yujia Chi1, Xiaoyu Zhai1, Han Jin2, Ziping Wang1*
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Thoracic Medical Oncology, Peking University Cancer Hospital and Institute, China
2Department of Cardiology, Peking University First Hospital, China
Abstract
Background: Cardiac and pericardial metastasis in small cell lung cancer (SCLC) is more common than estimated, but there are a few related studies in the literature. This study aims to raise the attention of such clinical circumstances.
Methods: We analyzed the clinical data of 62 SCLC patients with arrhythmia and confirmed eleven cases of SCLC with cardiac and/or pericardial metastasis by cytology or imaging diagnosis. Survival analysis was performed by the Kaplan-Meier method.
Results: Among 11 patients, 6 had pericardial involvement, 10 had mediastinal lymph node metastasis, and 8 had hilar lymph node metastasis. The most common type of electrocardiogram (ECG) abnormality was supraventricular arrhythmias (10/11). Complete imaging data were obtained in 7 patients through whole treatment after diagnosed with cardiac metastasis. Among them, 5 patients achieved partial response, and 2 of them achieved improvements in ECG abnormality. In the two remaining patients, advances in imaging diagnosis were identified after treatment, and new abnormalities were found in their ECG. The median overall survival time of the 11 patients was 11 months.
Conclusions: Cardiac and pericardial metastasis of SCLC can present different types of arrhythmia, and the ECG may change after treatment. Clinicians should take this condition into consideration, and aggressive treatment may achieve significant remission.
Introduction
Small cell lung cancer (SCLC), accounting for 15% of lung cancer patients, develops rapidly and is highly sensitive to chemotherapy. A total of 70% of the patients had already advanced to systemic metastasis when they were initially diagnosed1, and a few of them were confirmed to have heart involvement. Tamura A, et al.2 reported that 31% of lung cancer patients presented with cardiac metastasis; Abe S, et al.3, 44.4%; both were proven by autopsies. Studies have indicated that 7.5-17.9% of such patients were SCLC2-4. The gold standard is to detect the tumor cells in pericardial effusion or carry out cardiac biopsy. Images such as computed tomography (CT) and cardiac magnetic resonance, as well as electrocardiogram (ECG) may also provide clues. A recent prospective study found non-sustained ventricular tachycardia had a higher prevalence rate, as well as a worse prognosis, in unselected non-small cell lung cancer patients5. Previous studies found patients with heart metastasis, which were confirmed by biopsies, have a higher incidence of abnormal ECG2,6,7. However, clinicians often overlook the relevant diagnosis, as most patients remain clinically silent, and they do not check for heart metastasis during the diagnostic process. Therefore, the present study retrospectively analyzed the clinical data of 62 SCLC patients with arrhythmia, confirmed 11 cases of SCLC with cardiac and/or pericardial involvement by imaging, and explored their clinical characteristics to raise the attention of diagnosing heart metastasis in SCLC and then beginning effective treatment. We present the following article in accordance with the STROBE reporting checklist.
Materials and Methods
We analyzed a database of 62 Chinese SCLC patients with arrhythmia—all had evidence of pathology and abnormal ECG recordings—from January 2007 to December 2020 in Beijing Cancer Hospital. Ultimately, eleven cases were determined to have heart involvement; one of them was confirmed by cytological examination obtained from the pericardial effusion. Chest CT (GE lightspeed VCT 64-slice spiral scanner) enhancement imaging of the other 10 cases indicated that the tumor invaded the pericardium or heart structure. The remaining 51 patients were excluded due to lack of clear cytological or radiologic evidence. We assessed those 11 cases regarding clinical characteristics, imaging and ECG alterations (all data were obtained from 10 seconds 12-lead ECG and 24-hour ECG recordings in our hospital), the healing processes, and outcomes. Clinical efficacy was evaluated using Response Evaluation Criteria in Solid Tumors Version 1.1 through the CT images. Follow-up was made through telephone calls. Overall survival (OS) was defined as the period from initial chemotherapy to death caused by SCLC or the last follow-up date. SPSS (RRID:SCR_002865) version 26.0 was used to perform the Kaplan-Meier survival analysis and the survival difference between the two groups was compared using a log-rank test.
Results
Clinical characteristics:
Among the 62 patients, 11 were diagnosed with cardiac and/or pericardial metastasis based on clinical evidence. There was no significant difference in sex and age among patients with and without heart invasion as indicated in Table 1. Table 2 shows the clinical characteristics, primary lesion distribution, peripheral tissue involvement, symptoms and treatment of the 11 cases. All but one were at the extensive stage when diagnosed with SCLC. Most patients had evidence of metastasis in mediastinal lymph nodes (10/11) and hilar lymph nodes (8/11). Over half of these patients (8/11) presented with mediastinal involvement, and 7 of 8 had involvement of great vessels. The pericardium was the most vulnerable site, followed by the atrium and/or the ventricle. The imaging manifested as pericardial effusion or thickening or an unclear boundary between the tumor and the heart. The clinical symptoms varied from no complaints to syncope or shortness of breath. Only five patients were administered with echocardiography. Apart from one patient whose left ventricular ejection fraction could not be accurately measured due to atrial fibrillation (AF), another four patients had no significant impairment of cardiac function. The clinical data revealed that nine patients received first-line chemotherapy, and two patients initiated second-line, one of them changed to topotecan due to heart metastasis, the other failed second-line chemotherapy and manifested as pericardial involvement.
Table 1: Age and sex distribution.
|
|
Male |
Female |
Range of age |
Median age |
|
Cardiac metastasis |
11 |
3 |
45-77 |
60.93 |
|
Non-cardiac metastasis |
39 |
9 |
35-79 |
60.44 |
|
Total |
50 |
12 |
35-79 |
60.55 |
Table 2: Clinical characteristic of 11 SCLC with cardiac metastasis.
|
|
No. |
% |
|
Stage |
|
|
|
Limited stage |
1 |
9.09 |
|
Extensive stage |
10 |
90.91 |
|
Site of primary lesion |
|
|
|
Right upper lobe |
3 |
27.27 |
|
Right middle lobe |
3 |
27.27 |
|
Right lower lobe |
1 |
9.09 |
|
Left upper lobe |
2 |
18.18 |
|
Left lower lobe |
1 |
9.09 |
|
Right hilar |
1 |
9.09 |
|
Left hilar |
0 |
0.00 |
|
Involvement of surrounding structure |
|
|
|
Mediastinal lymph node |
10 |
90.91 |
|
Hilar lymph node |
8 |
72.73 |
|
Carina lymph node |
3 |
27.27 |
|
Peripheral vessel |
7 |
63.64 |
|
Mediastinum |
8 |
72.73 |
|
Involvement of heart structure |
|
|
|
Left atrium |
3 |
27.27 |
|
Right atrium |
3 |
27.27 |
|
Right ventricle |
1 |
9.09 |
|
Pericardium |
6 |
54.55 |
|
Clinical symptom |
|
|
|
Shortness of breath |
7 |
63.64 |
|
Syncope |
1 |
9.09 |
|
Asymptomatic |
3 |
27.27 |
|
Treatment |
|
|
|
First-line |
9 |
81.82 |
|
Second-line |
2 |
18.18 |
Electrocardiogram abnormalities:
Table 3 summarizes the ECG abnormalities of these 11 patients. 7 patients already had cardiac and/or pericardial metastasis as well as arrhythmia at initial diagnosis, while the rest 4 patients, who developed heart invasion during the course, only had non-specific ECG abnormalities at the beginning. Supraventricular arrhythmia was the most common abnormality, accounting for 90.91%, among which AF has the highest incidence rate, followed by supraventricular tachycardia (SVT) (excluding AF). Then came non-specific change, including ST-T changes, low voltage of limb leads, and left or right axis deviation. Three patients had conduction block: one patient had a I° atrioventricular block (AVB), and two patients had right bundle branch block (RBBB).
Table 3:ECG abnormalities
|
ECGa changes |
No. |
% |
|
Arrhythmia |
11 |
100.00 |
|
Supraventricular arrhythmia |
10 |
90.91 |
|
Supraventricular premature |
9 |
81.82 |
|
SVTb |
6 |
54.55 |
|
AFc |
8 |
72.73 |
|
Ventricular premature |
5 |
45.45 |
|
Sinus tachycardia |
4 |
36.36 |
|
Sinus bradycardia |
2 |
18.18 |
|
Non-specific changes |
6 |
54.55 |
|
ST-T wave changes |
3 |
27.27 |
|
Low voltage |
2 |
18.18 |
|
Right axis deviation |
1 |
9.09 |
|
Left axis deviation |
1 |
9.09 |
|
Conduction block |
3 |
27.27 |
|
RBBBd |
2 |
18.18 |
|
AVBe |
1 |
9.09 |
a: electrocardiogram
b: supraventricular tachycardia
c: atrial fibrillation
d: right bundle branch block
e: atrioventricular block
Treatment and outcome:
Imaging data for seven patients were complete, and could be used to assess the clinical outcomes (Table 4). All these patients received etoposide in combination with platinum as the first-line chemotherapy. Two of them received radiotherapy during the treatment course. One patient was lost to follow-up after 115 months, and the remaining six patients had an OS range of 3-14 months. Three of them had cardiac metastasis and two had pericardial metastasis at the time of initial treatment, and all responded satisfactorily to chemotherapy, which presented as markedly reduced sizes of primary lesions and heart invasion. For the remaining two patients, one patient achieved partial response (PR) after first-line EC chemotherapy with sequential radiotherapy. However, pericardial metastasis soon appeared, and malignant tumor cells were identified in the pericardial effusion. Hence the second-line of single agent topotecan was performed, but with an unsatisfied outcome.
Table 4: Electrocardiographic, clinical outcomes, and survival time after treatment.
|
Patient No. |
Sex |
Age |
Treatment |
Evaluation |
ECG changes after treatment |
OSa |
|
1 |
F |
77 |
ECb |
PRc |
ï¼-ï¼ |
11 |
|
2 |
M |
57 |
EC |
PR |
AF disappeared, SVT frequency reduced |
10 |
|
3 |
M |
45 |
EC |
PR |
ï¼-ï¼ |
3 |
|
4 |
M |
55 |
EC |
PR |
ï¼-ï¼ |
11 |
|
5 |
M |
58 |
EC with concurrent radiotherapy |
PR |
AF disappeared, SVT frequency reduced, low voltage disappeared. |
Lost to follow up |
|
6 |
M |
59 |
EC with sequential radiotherapy→Topotecan |
PDd |
New onset AF and SVT, ST-T wave changes |
14 |
|
7 |
M |
60 |
EPe |
PD |
New onset AF and SVT, newly appeared low voltage |
4 |
a: OS: Overall survival
b: EC: Etoposide/carboplatin
c: PR: Partial response
d: PD: Progressive disease
e: EP: Etoposide/cisplatin.
Another case was not sensitive to the first-line EP treatment, and pericardial involvement occurred after four cycles of chemotherapy. Six out of seven patients experienced new ECG abnormalities when diagnosed with cardiac or pericardial metastasis, including AF, SVT, low voltage and ST-T change. Four patients had ECG changes after treatment. These arrhythmias improved in 2 of 5 patients who had good clinical outcomes (which were evaluated by imaging). Both patients had AF and SVT on initial admission. The 24-hour ECG revealed the disappearance of AF and a reduction of SVT frequency (183→2 beats per 24 hours, and the other recorded less than 1% per 24 hours after treatment). One patient had improvement in limb leads low voltage. Conversely, two patients who progressed after treatment developed new types of ECG abnormalities, including new-onset AF, SVT, and emerging low voltage and ST-T wave changes (Fig 1, Fig 2).
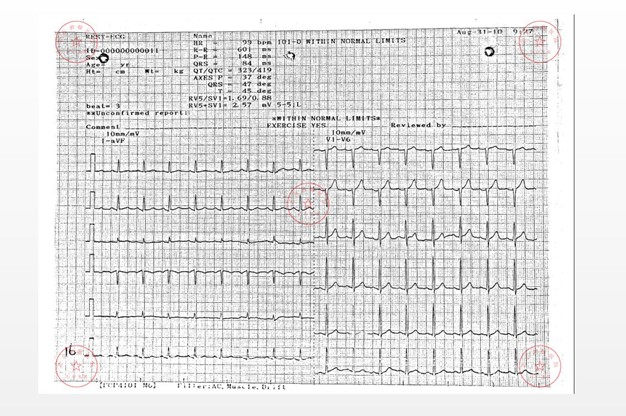
Figure 1a: Initial ECG in patient 6
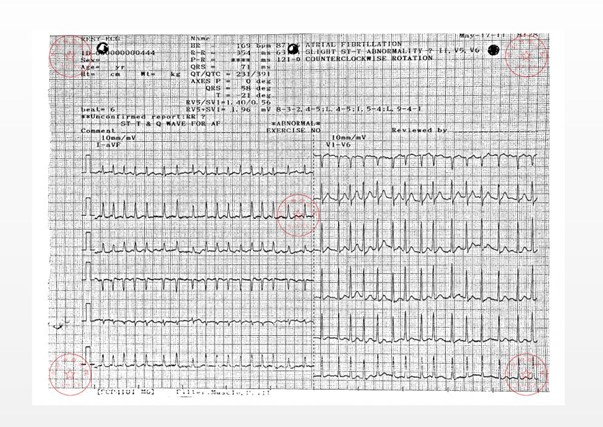
Figure 1a: Newly emerging ST-T wave changes in patient 6.
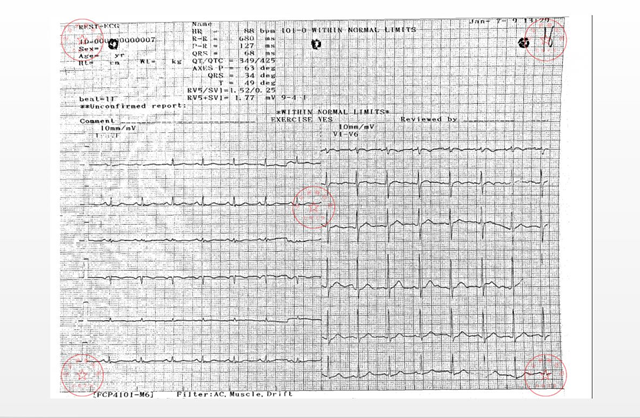
Figure 2a: Initial ECG in patient 7.
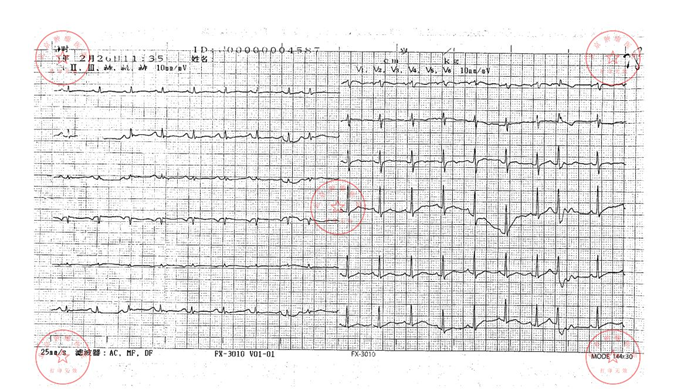
Figure 2b: Newly emerging limb leads low voltage in patient 7.
As of the end of the statistics, 2 of 11 patients with cardiac involvement were lost to follow-up, and the OS was calculated according to the last follow-up date. The other nine patients died of tumor. From the first chemotherapy date, the median OS of the patients who developed heart metastasis was estimated to be 11 months (95% CI: 9.1-12.9 months) (Fig 3), while those of non-heart metastasis was 12 months (95% CI: 9.7-14.3 months). There was no significant difference between the two groups (P>0.05).
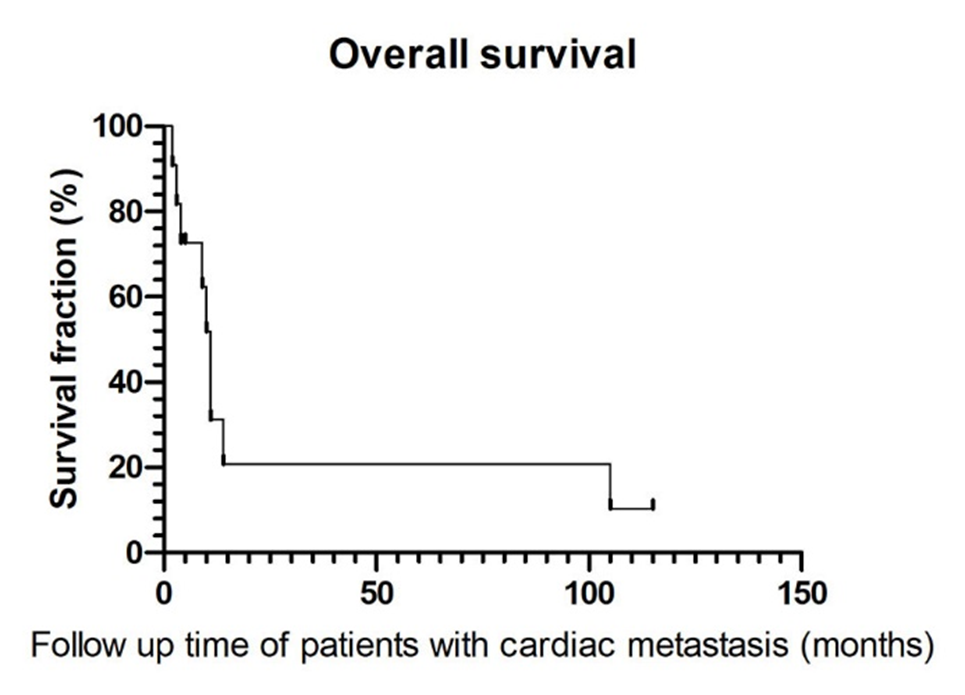
Figure 3: Survival curve of 11 patients with cardiac metastasis
Discussion
Heart metastasis of SCLC can initially be detected by echocardiography, and cardiac magnetic resonance is becoming the standard modality for further tissue evaluation8. But this phenomenon tends to be underestimated, as most patients are asymptomatic or experience atypical symptoms masked by the primary disease and did not go over relevant examinations. Among these analyzed patients, most had no specific symptoms, apart from one who had syncope, which might correlate to the arrhythmia caused by pericardial involvement. None of the patients who underwent echocardiographs presented with a decreased left ventricular ejection fraction. These findings indicate that early heart metastasis does not affect cardiac function. We should raise attention and diagnose it in a timely manner with the help of clinical symptoms and examinations.
Pericardium is the most susceptible site of cardiac involvement, followed by the myocardium and endocardium9,10. The lymphatics is the most common pathway to invade the pericardium, including the retrograde and left atrium-subcarinal lymphatic routes2,11. Tamura A, et al.2 also suggested a unique way that lung cancer can metastasize through the hilar lymph nodes; thus, even patients with lymph node metastasis in N1 stage should consider the possibility of pericardial infiltration. In addition, direct invasion of the original lesion or the hematogenous pathway are also routes for heart metastasis. As is shown in Table1, pericardium involvement accounted for the highest proportion (6/11), among which, 5 patients had hilar lymph node metastasis. Eight patients presented with primary focus directly invading the mediastinum. In conclusion, we need to consider the possibility of tumor disseminate to the heart through the lymphatic routes or directly invasion in patients with relevant lymph nodes and mediastinal involvement.
Previous studies have reported that there is a correlation between cardiac metastasis and abnormal ECG changes. Pericardial involvement can lead to pericardial tamponade. Myocardial metastasis can result in a conduction block and different types of arrhythmia10,12. If a tumor compresses the coronary artery, the ECG may manifest as ST-T wave abnormalities or even myocardial infarction changes7,13. Through analysis of the present recorded ECG, supraventricular and ventricular premature beats are most common non-specific manifestations, which can be detected regardless of the presence of a basic heart disease. However, when the ventricular premature beat is polymorphic, or is associated with non-sustained ventricular tachycardia, this may suggest a higher risk of mortality, new on-set heart failure, and so on14-16. In addition to premature beats, AF accounted for a substantial part of arrhythmia in 11 patients. Although many cardiovascular diseases and non-cardiac factors, such as electrolyte disorder, hypoxia, anemia, endocrine, drugs and other factors, can cause AF, rare causes include tumor compression or invasion of the heart or pulmonary vein17,18. Patients with new-onset AF have a higher risk of cancer, especially within 90 days after the initial diagnosis of AF19. In turn, the incidence of AF increases after cancer diagnosis, possibly due to the tumor-related systemic inflammation, anti-tumor treatment, or comorbid states20. Among the eight patients with AF, seven manifested new-onset AF after heart or pericardial involvement was found. Except for one patient who was undergoing chemotherapy and one who had hyperkalemia when AF occurred, all remaining patients had no related risk factors that can cause AF. Half of the present analyzed cases also recorded an onset of SVT. The common pathogeneses of SVT include reentry, enhanced automaticity and trigger activity. The repetitive tachycardia observed in adults may be correlated to organic heart disease and is sometimes observed in its terminal stage21. For cancer patients with arrhythmia, we need to suspect cardiac or pericardial involvement in those with a significant variation in the number of premature beats or SVT after the treatment. On the premise of excluding the influence of other factors, the correlation between newly diagnosed tumors and new-onset arrhythmia should be considered, especially for patients with imaging indicating cardiac infiltration.
In addition, half of these patients had nonspecific ECG abnormalities. Low QRS voltage, defined as the sum of QRS amplitudes of <5 mm in the limb leads, is often correlated to the process of electrical signals from their generation in the heart to conduction to the skin electrodes. The QRS voltage may be affected by myocardial infiltrative disease, pericardial infiltration, chronic obstructive pulmonary disease, and obesity22. The prevalence rate of RBBB and I° AVB increase with age, and patients without symptoms do not need treat. RBBB has no significant association with cardiac disease, while I°AVB has a higher risk of developing AF23,24. Non-specific ST-segment changes may be related to two causes: repolarization discordance due to bundle branch block, or improper measurement of ST-segment amplitude due to AF25. Therefore, a change in QRS voltage may have certain pathological significance, while ST-T changes may secondary to arrhythmia. For those without structural heart disease, dynamic ECG changes provide hints for heart metastasis.
The standard care of first-line chemotherapy for SCLC is platinum-based doublet-treatment (cisplatin or carboplatin combined with etoposide). In limited stages, standard chemotherapy combined with concurrent thoracic radiotherapy yields an objective response in 90% of patients. In extensive stages, 60-70% of patients achieve an objective response with chemotherapy26. There is no significant difference in efficacy between cisplatin or carboplatin treatment1,26. Seven patients had complete imaging data through the whole course of their disease. All received standard first-line chemotherapy. Although five of them had heart metastasis at the initial treatment, they all responded well, suggesting that even patients with heart invasion can be controlled through treatment. After chemotherapy, the ECG abnormalities of two patients improved along with the reduction of cardiac lesions, while 2 patients progressed with the occurrence of pericardial metastasis. The heart infiltration affects the electrophysiological activity, and further triggers different types of arrhythmia. In patients who only received anti-tumor therapy, improved or newly appearing ECG changes may be hints for undetected cardiac metastasis. Timely prediction and active treatment may have a better therapeutic effect.
Table 5 summarizes the previously reported SCLC cases with heart involvement. Two patients received chemotherapy, and one patient received cardiac radiotherapy. All three of these patients achieved improvement, including relief of clinical symptoms, improved imaging or ECG abnormalities. One patient received surgical treatment and adjuvant chemotherapy, but the outcome of this patient was not mentioned in the report. In another patient who experienced sudden death with no definite past history, and pericardial infiltration of SCLC was found by autopsy. It was speculated that the pericardial tamponade led to the sudden death. Two patients passed away before receiving treatment27-33. Previous cases suggested a potential benefit of receiving treatment after diagnosed with heart involvement in SCLC patients. SCLC is sensitive to chemotherapy and radiotherapy. Thoracic radiotherapy may cause early cardiac events, such as pericardial effusion, valvular disease, and atrial arrhythmia, which were associated with worse OS34. However, when patients suffer from acute cardiovascular events, such as pericardial tamponade, it is still a reasonable treatment approach to relieve the clinical symptoms31. Quraishi, M. A, et al.4 reported that in patients with pericardial metastasis, those receiving treatment had a longer OS than those who did not. These findings serve as a reminder that though diagnosed with cardiac or pericardial metastasis, active treatment can lead to a better prognosis.
The present study had some limitations. First, the sample size was too small to compare the OS between patients whether or not they received treatment, or had different treatments. Second, none of these patients underwent cardiac magnetic resonance or biopsy leading to defects of clinical evidence in these patients. Additionally, diagnostic suspicion bias on the ECG changes may exist once cases present with cardiac or pericardial involvement. Therefore, more clinical data is needed to support these assumptions.
Conclusion
SCLC cardiac and/or metastasis is not uncommon. The key to its diagnosis is to be aware of the association of heart metastasis in SCLC; the clinician should not simply attribute the relevant clinical manifestations to previous heart diseases. Many patients are clinically silent, but objective examinations, such as imaging and ECG, could provide clues. We should carefully investigate the patients with ECGs presenting with new arrhythmia or pericardial tamponade-like changes, such as low voltage and alternating electric heart axis, especially those imaging modalities indicating pericardial effusion or thickening. It is necessary for physicians to combine ECG, cardiac imaging, pathology and other objective examination methods to form a comprehensive judgment. When necessary, this can be assisted by myocardial magnetic resonance and myocardial biopsy. Chemotherapy is still very sensitive to SCLC patients with cardiac metastasis; however, given the cardiotoxicity of these drugs, great care should be taken when choosing the chemotherapy regimen for patients with arrhythmia, in order to avoid the risk of cardiac toxicity resulting from the drugs, and an awkward situation that is difficult to explain after the emergence of new arrhythmias. Clinical experience has shown that for patients who are sensitive to chemotherapy, ECG improvement can be observed soon after the treatment. Therefore, active treatment should be applied for these patients. Clinicians could also consider thoracic radiotherapy and monitor the symptoms, signs, ECGs and imaging changes.
Acknowledgement
This work was supported by Science Foundation of Peking University Cancer Hospital [18-02]; Capital Clinical Characteristics and Application Research [Z181100001718104]; and Beijing Excellent Talent Cultivation Subsidy Young Backbone Individual Project [2018000021469G264].
References:
- Wang Y, Zou S, Zhao Z, et al. New insights into small-cell lung cancer development and therapy. Cell Biol Int. 2020; 44:1564-1576.
- Tamura A, Matsubara O, Yoshimura N, et al. Cardiac metastasis of lung cancer. A study of metastatic pathways and clinical manifestations. Cancer. 1992; 70:437-442.
- Abe S, Watanabe N, Ogura S, et al. Myocardial metastasis from primary lung cancer: myocardial infarction-like ECG changes and pathologic findings. Jpn J Med. 1991;30:213-218.
- Quraishi MA, Costanzi JJ, Hokanson J. The natural history of lung cancer with pericardial metastases. Cancer. 1983; 51:740-742.
- Anker MS, von Haehling S, Coats AJ, et al. Ventricular tachycardia, premature ventricular contractions, and mortality in unselected patients with lung, colon, or pancreatic cancer: a prospective study. Eur J Heart Fail. 2020.
- Wadler S, Chahinian P, Slater W, et al. Cardiac abnormalities in patients with diffuse malignant pleural mesothelioma. Cancer. 1986; 58:2744-2750.
- Nakamura A, Suchi T, Mizuno Y. The effect of malignant neoplasms on the heart. A study on the electrocardiographic abnormalities and the anatomical findings in cases with and without cardiac involvement. Jpn Circ J. 1975; 39:531-542.
- Bilani N, Elson L, Martinez F, et al. A Multimodal Approach to Evaluate for Cardiac Metastasis in a Case of Non-Small Cell Lung Cancer. Case Rep Oncol. 2020; 13:212-218.
- Moazez C, Howard E, Mehdizadeh A, et al. Intracardiac Metastasis as the Initial Presentation ofNon-Small Cell Lung Cancer. Am J Med. 2018; 131:e502-e503.
- Haq S, Roomi S, Lashari BH, et al. Non-Sustained Ventricular Tachycardia as a Sign of Lung Cancer. Cureus. 2019; 11:e6090.
- Kline IK. Cardiac lymphatic involvement by metastatic tumor. Cancer. 1972; 29:799-808.
- Kinoshita K, Hanibuchi M, Kishi M, et al. Case of squamous cell lung cancer with myocardial metastasis complicated with ventricular tachycardia. Nihon Kokyuki Gakkai Zasshi. 2009; 47:817-822.
- WiBt T, Jehn CF, Vierbuchen M, et al. Solitary neuroendocrine carcinoma of the heart: a case report. Eur Heart J Case Rep. 2018; 2:yty096.
- Corrado D, Drezner JA, D'Ascenzi F, et al. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med. 2020; 54:1142-1148.
- Lin CY, Chang SL, Lin YJ, et al. Long-term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol. 2015; 180:80-85.
- Lin CY, Chang SL, Chung FP, et al. Long-Term Outcome of Non-Sustained Ventricular Tachycardia in Structurally Normal Hearts. PLoS One. 2016; 11:e0160181.
- Ahmed N, Carlos MM, Moshe G, et al. Association Between Left Atrial Compression And Atrial Fibrillation: A Case Presentation And A Short Review Of Literature. J Atr Fibrillation. 2016; 9:1458.
- Tamura M, Kaneko Y, Nakajima T, et al. A case of atrial tachycardia originating from pulmonary vein invaded by lung cancer. J Cardiol Cases. 2012; 5:e118-e121.
- Vinter N, Christesen AMS, Fenger-Grøn M, et al. Atrial Fibrillation and Risk of Cancer: A Danish Population-Based Cohort Study. J Am Heart Assoc. 2018; 7:e009543.
- Chu G, Versteeg HH, Verschoor AJ, et al. Atrial fibrillation and cancer - An unexplored field in cardiovascular oncology. Blood Rev. 2019; 35:59-67.
- Levine HD, Smith C Jr. Repetitive paroxysmal tachycardia in adults. Cardiology. 1970; 55:2-21.
- Kim DH, Verdino RJ. Electrocardiogram voltage discordance: Interpretation of low QRS voltage only in the precordial leads. J Electrocardiol. 2017; 50:551-554.
- Harkness WT, Hicks M. Right Bundle Branch Block. 2020, StatPearls Publishing LLC; 2020.
- Oldroyd SH, Quintanilla Rodriguez BS, Makaryus AN. First Degree Heart Block. StatPearls Publishing LLC; 2020.
- Rivero D, Alhamaydeh M, Faramand Z, et al. Nonspecific electrocardiographic abnormalities are associated with increased length of stay and adverse cardiac outcomes in prehospital chest pain. Heart Lung. 2019; 48:121-125.
- Kalemkerian GP, Schneider BJ. Advances in Small Cell Lung Cancer. Hematol Oncol Clin North Am. 2017; 31:143-156.
- Lin MT, Ku SC, Wu MZ, et al. Intracardiac extension of lung cancer via the pulmonary vein. Thorax. 2008; 63:1122.
- Shah R, John E, Fan TH, et al. A Patient with Metastatic Small-Cell Lung Cancer and Giant Right Ventricular Mass. Echocardiography. 2016; 33:491-493.
- Papavdi A, Nathena D, Kranioti EF, et al. Advanced bronchogenic carcinoma presented as cardiac tamponade: a case report and a review of the literature. Am J Forensic Med Pathol. 2015; 36:13-15.
- Orcurto MV, Delaloye AB, Letovanec I, et al. Detection of an asymptomatic right-ventricle cardiac metastasis from a small-cell lung cancer by F-18-FDG PET/CT. J Thorac Oncol. 2009; 4:127-130.
- Chen CF, Lin MH, Chu KA, et al. Effective cardiac radiotherapy relieved life-threatening heart failure caused by advanced small cell lung cancer with cardiac metastasis: a case report. J Thorac Dis. 2018; 10:E250-e254.
- Pham N, Bonnen MD, Ghebre YT. Silent Neoplastic Cardiac Invasion in Small Cell Lung Cancer: A Case Report and Review of the Literature. Am J Case Rep. 2018; 19:619-622.
- Hammerschmidt S, Pohlink C, Wirtz H, et al. Alternating electric heart axis in a patient with small cell lung cancer. Dtsch Med Wochenschr. 2004; 129:19-22.
- Borkenhagen JF, Bergom C, Rapp CT, et al. Dosimetric Predictors of Cardiotoxicity in Thoracic Radiotherapy for Lung Cancer. Clin Lung Cancer. 2019; 20:435-441.
