Evaluation of structural myocardial changes during chronic hypertensive states in rats
Alexandar A. Iliev1*, Georgi N. Kotov1, Iva N. Dimitrova2 and Boycho V. Landzhov1
1Department of Anatomy, Histology and Embryology, Medical University of Sofia, Bulgaria
2Department of Cardiology, University Hospital ‘St. Ekaterina’, Medical University of Sofia, Bulgaria
Abstract
Introduction: Spontaneously hypertensive rats are often used as a model of arterial hypertension in humans. Cardiomyocytic hypertrophy, focal myocytolysis and ventricular fibrosis are only a part of the alterations in the morphology of the myocardium observed in spontaneously hypertensive rats with the progression of hypertension. The present manuscript reviews our studies on spontaneously hypertensive rats, with focus on the microscopic changes in the myocardial architectonics and analysis of several morphometric parameters.
Materials and methods: A total of 12 male spontaneously hypertensive rats, distributed in two age groups, each containing six animals: 1-month-old (young) and 6-months-old (adult) were used. We also used 12 male normotensive Wistar rats, distributed in two age groups, each containing six animals: 1-month-old (young) and 6-months-old (adult). Routine haematoxylin and eosin staining and Mallory’s trichrome stain were conducted. Quantitative data were obtained with a computerized system for image analysis NIS-Elements Advanced Research (Ver. 2.30).
Results: Changes in the normal morphology of the myocardium included cardiomyocytic hypertrophy, focal myocytolysis and ventricular fibrosis. As aging progressed, we noted a significant increase in the thickness of the free wall and cross-sectional area of the cardiomyocytes and the cardiomyocytic nuclei and a decrease in cardiomyocytic density.
Conclusion: The manuscript presents a detailed qualitative and quantitative study of changes in the normal structure of the myocardium initiated by arterial hypertension.
Introduction
Arterial hypertension has a profound effect on the morphology and function of the myocardium. It is often associated with increased morbidity from atherosclerotic disease, stroke and multiple other cardiovascular and cerebrovascular events1. Spontaneously hypertensive rats (SHR) are often used as a model of arterial hypertension in humans and allow researchers to study and modify the connection between hypertension as the initiating factor and alterations in myocardial morphology and function as a consequence to it2-4. Cardiomyocytic hypertrophy, focal myocytolysis and ventricular fibrosis are only a part of the alterations in the morphology of the myocardium observed in spontaneously hypertensive rats with the progression of hypertension. They, in turn, lead to impaired ventricular relaxation and decreased cardiac output3,5. Several studies report an enlarged myocyte cross section, as well as an increase in the collagen content of the myocardium6,7. They conclude that the remodeling of the left ventricle is a result of the concomitant presence of molecular, cellular, and biochemical events9.
Several studies have tried to assess the quantitative effect that hypertension exerts on the myocardium but most have been limited only to measurements of the cellular diameter or the cross-sectional area; some researchers have tried to measure the length of the cardiomyocyte8-11. Bishop et al.12 found that the diameter of cells from a papillary muscle in the left ventricle is longer than that of cells in the free wall in young SHR; this trend is reversed after the 21st week (at age 5 months). Another study discovered that the cross-sectional area of subendocardial cardiomyocytes from normotensive rats is larger; conversely, under hypertensive conditions, subepicardial become larger than subendocardial cells13. The same study reported that the cellular length is longer in subepicardial cells than in subendocardial cells both in normotensive rats and SHR13.
The aim of the present article is to study spontaneously hypertensive rats, with focus on the microscopic changes in the myocardial architectonics and analysis of several morphometric parameters – thickness of the free wall (TFW) of the left and right ventricle; cross-sectional area (CSA) of the cardiomyocytes and of the cardiomyocytic nuclei and cardiomyocytic density. The parameters CSA of the cardiomyocytic nuclei and cardiomyocytic density were studied for the first time and no similarities were found in the literature.
Materials and methods:
In the present study, we used histological material from the hearts of twelve male SHR distributed in two age groups, each containing six animals: one-month-old (young) and six-months-old (adult). We also used twelve male normotensive Wistar rats (WR) for the purpose of the morphometric analysis, which were also distributed in two age groups, each containing six animals: one-month-old (young) and six-months-old (adult). The body weight and heart weight of the animals are presented in Table 1. All experiments were conducted with the approval of the University Committee on Animal Resources. The animals were anaesthetized with ether and cervical dislocation was performed. The hearts were removed from the chest cavity and were placed in physiological saline in order to rinse the blood in the cardiac cavities. After a short fixation in 10% neutral buffered formalin, a transverse section was performed through the middle of the heart along the coronary sulcus, a little below the level of the cardiac valves and parallel to the short axis of the heart, thus dividing it into two halves. The hearts were then placed again in 10% neutral buffered formalin for immersion fixation. Afterwards, they were fixed in paraffin blocks, from which we prepared 7 μm wide paraffin tissue slides. These slides represented a series of transverse cross-sections through the ventricles. Routine haematoxylin and eosin staining and Mallory’s trichrome stain for specific visualization of collagen were conducted.
Table 1. Mean body weight and heart weight of 1- and 6-month-old spontaneously hypertensive rats (SHR) and 1- and 6-month-old normotensive Wistar rats (WR).
| Age group | Body weight (g) | Heart weight (g) |
|---|---|---|
| 1-month-old SHR | 79.6 ± 1.8 | 0.38 ± 0.01 |
| 1-month-old WR | 88.3 ± 2.2 | 0.32 ± 0.02 |
| 6-month-old SHR | 420.4 ± 3.1 | 1.70 ± 0.06 |
| 6-month-old WR | 361.5 ± 2.8 | 1.35 ± 0.08 |
The morphometric analysis was performed on five slides from the heart of each animal. Quantitative data were obtained with a computerized system for image analysis NIS-Elements Advanced Research (Ver. 2.30). The areas of interest in each slide were first found on low magnifications (x100, x200), taking into account the respective age group. Afterwards, at magnification x400, we photographed an area of the myocardium, containing transversely cut cardiomyocytes, and a zone with a surface area of 0.04 mm2 was marked. The borders of the cardiomyocytes within the zone were outlined. Afterwards, the following morphometric parameters were obtained automatically: thickness of the free wall of the left and right ventricle (μm); cross-sectional area of the cardiomyocytes (μm2); cross-sectional area of the cardiomyocytic nuclei (μm2); cardiomyocytic density – calculated as number of cells per unit of surface area of the slide (number of cells / mm2). The obtained quantitative data were statistically evaluated through a Student-T-test. Statistically significant differences between the SHR and the corresponding age groups of normotensive WR in the left and right ventricle were read in the case of p<0.05.
Results:
The analysis of histological slides routinely stained with haematoxylin and eosin in the group of young SHR revealed a normal morphological appearance of the myocardium. The cardiomyocytes presented as eosinophilic cells, with well-expressed cross striations and one or two centrally positioned basophilic nuclei. The cells branched and formed a complex reticular network. Each individual cardiac muscle cell was enveloped by endomysium made up of thin connective tissue and perimysium with a relative abundance of capillaries and collagen fibers. We noted the presence of fibroblastic and fibrocytic nuclei, which appeared more flattened and more basophilic than the cardiomyocytic nuclei. On a transverse section, capillaries often presented as ‘empty’ oval structures; on a higher magnification, we sometimes noted a thickening of their wall marking the location of the nucleus of the endothelial cell (Figure 1a). In the group of 6-month-old SHR, this normal architectonics was significantly altered. We noted the narrowing of the ‘empty’ spaces, i.e. the lumens of the capillaries, which was interpreted as cardiomyocytic hypertrophy. Conversely, the connective tissue fibers were clearly visualized and presented as eosinophilic fascicles located among the cardiomyocytes, predominantly in the subendocardial and interstitial zone. The number of binucleate cardiomyocytes increased. We also described the phenomenon of focal myocytolysis – numerous cardiomyocytes appeared ‘empty’, i.e. had no nuclei and no cross striations and their cytoplasm was more eosinophilic. Neutrophil infiltration was present, as well as pyknotic nuclei. These changes were better expressed in slides from the wall of the left ventricle, as opposed to the right ventricle, where the normal structure of the myocardium was relatively well preserved (Figure 1b).
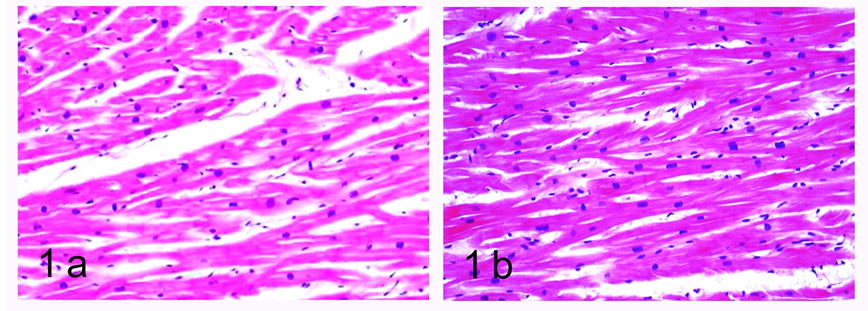
Figure 1: Light microscopic histological study of the myocardium in spontaneously hypertensive rats. Haematoxylin and eosin stain.
a. Left ventricle, age 1 month, magnification x200.
b. Right ventricle, age 6 months, magnification x200.
Mallory’s trichrome stain in 1-month-old SHR revealed an abundance of collagen fibers in the perivascular zones in the wall of the left ventricle (Figure 2a). They were stained intensively blue, had a complex spiral structure and formed a network, from which multiple fascicles extended into the interstitial space. In the wall of the right ventricle in this age group, collagen fibers were found almost exclusively in the perivascular zones, with only a small number of thin fibers present in the interstitial space (Figure 2b). In the group of adult SHR, Mallory’s trichrome stain in the left ventricle was dominated by blue colour. Collagen fibers were much thicker than in the group of young animals, had a complex spiral structure and stained intensively. They were present in the perivascular zone, extended into the interstitial space and occupied a bigger portion of it, surrounding individual cardiomyocytes (Figure 2c). In the right ventricle, we noted a lot more collagen fibers in the interstitial space compared to the group of 1-month-old SHR. They were relatively thick, spiral-shaped but stained less intensively than those in the left ventricle and did not occupy that much of the interstitial space (Figure 2d).
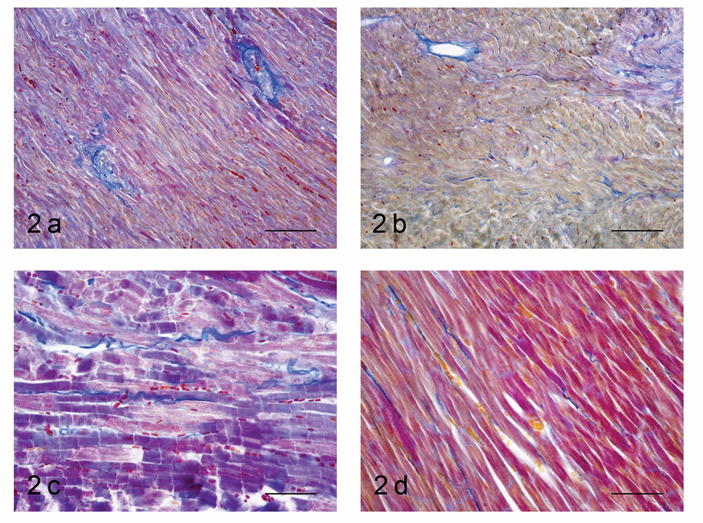
Figure 2: Light microscopic histological study of the myocardium in spontaneously hypertensive rats. Mallory’s trichrome stain.
a. Left ventricle, age 1 month, magnification x200.
b. Right ventricle, age 1 month, magnification x200.
c. Left ventricle, age 6 months, magnification x400.
d. Right ventricle, age 6 months, magnification x400.
The analysis of the morphometric parameters gave a numerical representation of the degree of growth of the myocardium, the individual cardiomyocytes and their nuclei depending on the degree of afterload of the respective ventricle. For visual representation of the descriptive statistics and comparison of left with right ventricles, young with adult animals and SHR with WR box plot diagrams are used (Figures 3-6).
The thickness of the free wall of the left ventricle increased over 40% in the group of 6-month-old SHR from the respective value for the group of 1-month-old animals. That increase was even more expressed in the right ventricle, where the parameter increased 60%. In both groups, however, the parameter had much higher values in the left ventricle – almost 3 times in the group of young SHR and over 2.5 times in the group of adult SHR. The rate at which the TFW of the left ventricle increased in young (1-month-old) SHR was comparable to that in adult (6-month-old) normotensive WR. In the right ventricle, in the group of 1-month-old animals, the values in SHR and normotensive WR were comparable, while in adult animals, the value of the TFW in SHR was more than 2 times that in normotensive animals (Table 2; Figure 3).
Table 2. Descriptive statistics for thickness of the free wall of the left and right ventricle in spontaneously hypertensive rats (SHR) and corresponding values in normotensive Wistar rats (WR).
| Age group | SHR | WR | T-test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean – μm | Standard deviation (SD) | MIN | MAX | Mean – μm | Standard deviation (SD) | MIN | MAX | ||
| 1-month-old, left ventricle | 2252.3 | 221.9 | 1978 | 2485 | 1702.7 | 17.6 | 1686 | 1726 | p<0.001 |
| 1-month-old, right ventricle | 775.1 | 52.7 | 713 | 834 | 561.4 | 81.9 | 500 | 673 | p<0.001 |
| 6-month-old, left ventricle | 3227.5 | 267.6 | 2906 | 3661 | 2596.1 | 146.7 | 2402 | 2736 | p<0.001 |
| 6-month-old, right ventricle | 1237.9 | 43.9 | 1196 | 1299 | 571.3 | 4.3 | 567 | 577 | p<0.001 |
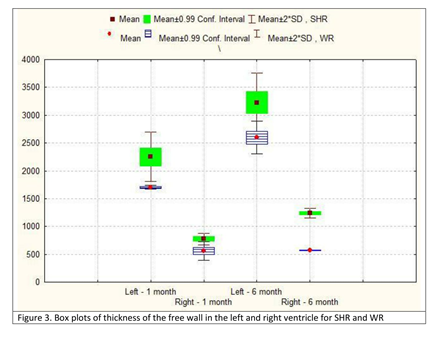
Figure 3: Box plots of thickness of the free wall in the left and right ventricle for SHR and WR.
SHR – spontaneously hypertensive rats; WR – Wistar rats; SD – standard deviation
The parameters cross-sectional area of the cardiomyocytes and cross-sectional area of their nuclei showed a similar increase in both age groups, both in SHR and normotensive WR. In 6-month-old SHR, compared to the group of 1-month-old animals, the value of the CSA of the cardiomyocytes increased roughly 2 times in the left ventricle and almost 3 times in the right ventricle. However, despite the increase being more significant in the right ventricle, the value of the parameter in the left ventricle of 1-month-old SHR was already 2 times higher than the value in the right ventricle of the same age group SHR and was 40% higher than the value in the right ventricle in the age group of 6-month-old SHR (Table 3; Figure 4). The parameter CSA of the cardiomyocytic nuclei revealed a similar pattern. We noted that the value in the left ventricle of 1-month-old SHR was comparable to that in both ventricles of a 6-month-old WR. The highest read value for this parameter was in the left ventricle of 6-month-old SHR, which was almost 2 times the value in the left ventricle of a 1-month-old SHR (Table 4; Figure 5).
Table 3. Descriptive statistics for cross-sectional area of the cardiomyocytes in the left and right ventricle in spontaneously hypertensive rats (SHR) and corresponding values in normotensive Wistar rats (WR).
| Age group | SHR | WR | T-test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean – μm2 | Standard deviation (SD) | MIN | MAX | Mean – μm2 | Standard deviation (SD) | MIN | MAX | ||
| 1-month-old, left ventricle | 315.3 | 315.3 37.7 | 297 | 330 | 214.7 | 35.3 | 195 | 250 | p<0.001 |
| 1-month-old, right ventricle | 158.8 | 18.1 | 144 | 173 | 65.8 | 12.6 | 55 | 78 | p<0.001 |
| 6-month-old, left ventricle | 652.7 | 69.2 | 642 | 672 | 490.1 | 55.9 | 475 | 523 | p<0.001 |
| 6-month-old, right ventricle | 457.4 | 51.6 | 439 | 475 | 223.6 | 39.2 | 198 | 247 | p<0.001 |
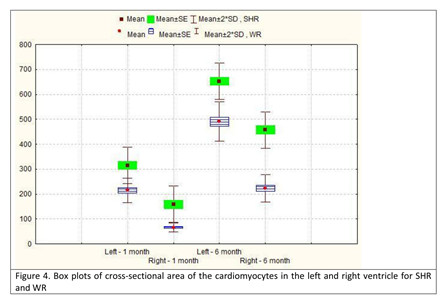
Figure 4: Box plots of cross-sectional area of the cardiomyocytes in the left and right ventricle for SHR and WR.
SHR – spontaneously hypertensive rats; WR – Wistar rats; SE – standard error; SD – standard deviation
Table 4. Descriptive statistics for cross-sectional area of the cardiomyocytic nuclei in the left and right ventricle in spontaneously hypertensive rats (SHR) and corresponding values in normotensive Wistar rats (WR).
| Age group | SHR | WR | T-test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean – μm2 | Standard deviation (SD) | MIN | MAX | Mean – μm2 | Standard deviation (SD) | MIN | MAX | ||
| 1-month-old, left ventricle | 16.6 | 1.6 | 13 | 22 | 10.1 | 1.2 | 8 | 18 | p<0.001 |
| 1-month-old, right ventricle | 9.6 | 2.3 | 5 | 16 | 6.1 | 2.2 | 4 | 8 | p<0.001 |
| 6-month-old, left ventricle | 29.4 | 2.1 | 21 | 44 | 17.3 | 17 | 11 | 25 | p<0.001 |
| 6-month-old, right ventricle | 16.7 | 1.8 | 9 | 19 | 11.9 | 2.7 | 8 | 16 | p<0.001 |
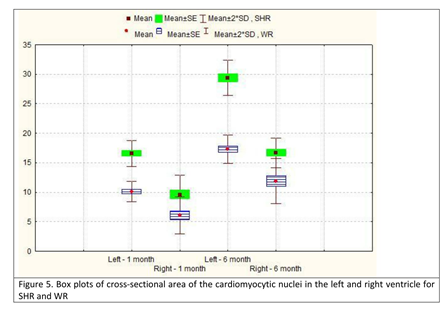
Figure 5: Box plots of cross-sectional area of the cardiomyocytic nuclei in the left and right ventricle for SHR and WR.
SHR – spontaneously hypertensive rats; WR – Wistar rats; SE – standard error; SD – standard deviation
Changes in the parameter cardiomyocytic density from both ventricles were inversely proportional to those observed for the CSA. In young SHR, the value was higher in the left as opposed to the right ventricle; however, at age 6 months, this tendency was reversed and the value in the right ventricle was more than 20% higher in the right as opposed to the left ventricle. The same trend was observed in normotensive WR. In each age group, for each ventricle, the parameter was always higher in normotensive WR. We noted that the parameter in cells from the left ventricle in 1-month-old SHR was not comparable to that in 6-month-old WR, being significantly higher. In the right ventricle, however, the parameter in the right ventricle of a 6-month-old WR was higher than the value in the right ventricle of a 1-month-old SHR (Table 5; Figure 6).
Table 5. Descriptive statistics for cardiomyocytic density in the left and right ventricle in spontaneously hypertensive rats (SHR) and corresponding values in normotensive Wistar rats (WR).
| Age group | SHR | WR | T-test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean – cells/mm2 | Standard deviation (SD) | MIN | MAX | Mean – μm2 | Standard deviation (SD) | MIN | MAX | ||
| 1-month-old, left ventricle | 1567.1 | 108.6 | 1336 | 1789 | 1987.3 | 150.9 | 1850 | 2200 | p<0.001 |
| 1-month-old, right ventricle | 1159.5 | 109.6 | 1086 | 1303 | 1468.6 | 55.9 | 1400 | 1525 | p>0.05 |
| 6-month-old, left ventricle | 624.8 | 128.3 | 550 | 667 | 880.7 | 65.2 | 720 | 975 | p<0.001 |
| 6-month-old, right ventricle | 773.7 | 151.5 | 758 | 809 | 1237.1 | 208.1 | 1050 | 1525 | p<0.001 |
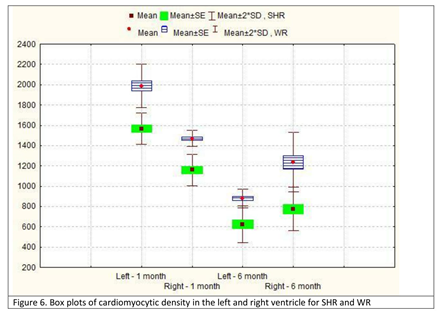
Figure 6: Box plots of cardiomyocytic density in the left and right ventricle for SHR and WR.
SHR – spontaneously hypertensive rats; WR – Wistar rats; SE – standard error; SD – standard deviation
Discussion:
The present study found an increase in the morphometric parameters TFW and CSA of the cardiomyocytes and their nuclei and a decrease in the parameter cardiomyocytic density. We also reported an increase in the collagen content of the myocardium in older animals, as well as presence of cardiomyocytic hypertrophy, focal myocytolysis, neutrophil infiltration and an increase in the number of binucleate cardiomyocytes.
Anversa et al.14 reported that a significant loss of muscle fibers was observed in both ventricles in normotensive rats. Our findings support those observations and further discover that age-related loss of cardiac muscle cells, assessed on the basis of the decrease in cardiomyocytic density with age is observed in SHR. Anversa et al.14 also showed that cellular hyperplasia too constitutes a response of the myocardium to hypertension. Although these authors noted that cardiomyocytic loss in the two ventricles was comparable, cellular hyperplasia occurred at an accelerated rate in the left ventricle. Over the course of the studied 25-month interval, the muscle component increased 1.75 times in the right ventricle and 2.2 times in the left14. Our studies revealed that cardiomyocytic loss, ventricular fibrosis and compensatory hypertrophy of the remaining cardiomyocytes were less pronounced in the right ventricle. These differences arise from higher afterload experienced by the left ventricle under hypertensive conditions, which depletes its reserve capacity at a younger age.
Literature data support the hypothesis that nuclear hyperplasia is accompanied by a similar increase in the number of cells15,16. Our results revealed an increase in the parameter cross-sectional area of the cardiomyocytic nuclei both in SHR and normotensive WR, across all age groups; however, this increase was more pronounced in the groups of SHR. These findings were interpreted both as signs of nuclear hypertrophy, as well as possible nuclear hyperplasia, which was supported by our discovery of more binucleate cardiomyocytes in the group of adult animals as opposed to young SHR. In their study, Cury et al.17 investigated the length of the sarcomeres and several morphometric parameters of the mitochondria and concluded that although no significant reduction in the mitochondrial volume was observed, the length of the sarcomeres increased and the results for the mitochondrial area were quite variable. These data also confirm that the alterations in the myocardial structure are not only present at the macroscopic level but also within the very ultrastructure of the cardiac muscle cell.
Over a period of 45 days after birth, in the left ventricle, the cellular volume in SHR increases by 151% which is achieved through an increase in the cross-sectional area by 77% and a total increase in the longitudinal size of the cardiomyocytic population by 42%18. In contrast, the mass of the cardiomyocytes in normotensive rats increases by 124% which is a result of an increase in the cross-sectional area by 47% and an increase in the longitudinal size of the cells by 53%18. Other studies have concluded that the progressive increase in the diameter and cross-sectional area in SHR represents a consequence of the continuous increase in the systemic arterial pressure19,20. According to Wagner et al.21, the body weight in SHR and Wistar-Kyoto rats (WKY) at age 10-12 weeks was identical, however, the heart weight in SHR was 30% higher than that of WKY rats. These changes in the parameters may be interpreted as a compensatory mechanism of the myocardium at the cellular level, aimed at increasing the negative effects of the increased afterload under hypertensive conditions2. Our previous studies have shown that the myocardial response to increased stress includes ventricular remodeling with an accumulation of collagen and development of reactive fibrosis22, a specific location-targeted up-regulation of enzymes of the NOS group5,23,24 and a decrease in the capillary density25.
The findings reported in the present manuscript represent a detailed analysis of changes in the myocardium in WR and SHR with age. Some of the morphometric parameters (CSA of the cardiomyocytic nuclei and cardiomyocytic density) have been studied for the first time in the literature. The present study provides a basis for better understanding of the morphological aspect of pathological processes and can be used as a reference point for similar studies in humans.
Conclusions:
The values obtained for the studied morphometric parameters correspond to the development of cardiomyocytic hypertrophy (an increase in the TFW, CSA of the cardiomyocytes and their nuclei) as a response to focal myocytolysis (a decrease in the cardiomyocytic density). Apart from providing a numerical representation of these phenomena, they revealed the following trend: a more pronounced effect of hypertension on the left ventricle, with higher values for TFW and CSA and lower values for cardiomyocytic density in older animals; a steeper change in values, best reflected in the parameter cardiomyocytic density, which was higher in the right ventricle as opposed to the left ventricle in adult SHR. Further studies on the ultrastructure of cardiomyocytes and its pathological alterations can help in establishing more precise concepts for the treatment of arterial hypertension and other myocardial pathologies.
Acknowledgement
The authors of the present manuscript would like to thank Associated Professor Jordanka A. Angelova of the University of Chemical Technology and Metallurgy, Sofia, Bulgaria for her kind assistance in the preparation of the statistical analysis of the obtained results.
Conflict of Interest
The authors of the present manuscript hereby deny any conflict of interest.
References
- Karabinov V, Georgiev GP. Current concepts in the management of patients with extracranial carotid artery atherosclerotic disease. CP Case. 2017; 1(3): 013.
- Iliev AA, Kotov GN, Landzhov BV, et al. A comparative morphometric study of the myocardium during the postnatal development in normotensive and spontaneously hypertensive rats. Folia Morphol (Warsz) (in press), doi: 10.5603/FM.a2017.0094.
- Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiol Rev. 1993; 73: 725–64.
- Sen S. Regression of cardiac hypertrophy. Experimental animal model. Am J Med. 1983; 75: 87-93.
- Iliev A, Jelev L, Landzhov B, et al. An immunohistochemical study of the expression of neuronal NOS in the myocardium of spontaneously hypertensive rats. Compt Rend Acad Bulg Sci. 2017; 70(8): 1157-62.
- LeGrice IJ, Pope AJ, Sands GB, et al. Progression of myocardial remodeling and mechanical dysfunction in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2012; 303(11): H1353-65.
- Tran N, Giannakidis A, Gullberg GT, et al. Quantitative analysis of hypertrophic myocardium using diffusion tensor magnetic resonance imaging. J Med Imaging (Bellingham). 2016; 3(4): 046001.
- Iliev A, Kotov G, Landzhov B, et al. Quantitative characteristics of the myocardium and the cardiomyocytes during different stages of the postnatal development in Wistar rats. Scr Sci Med. 2016; 48(Suppl.2): 53.
- Iliev AA, Kotov GN, Landzhov BV, et al. A comparative quantitative analysis of the postnatal changes in the myocardium of the left and right ventricle in rats. Folia Med (Plovdiv) (in press), doi: 10.1515/folmed-2017-0089.
- Aherne W. A method of determining the cross-sectional area of muscle fibers. J Neurol Sci. 1968; 7: 509-28.
- Ashley LM. A determination of the diameters of myocardial fibers in man and other animals. J Anat. 1945; 77: 325-47.
- Bishop SP, Oparil S, Reynolds RH, et al. Regional myocyte size in normotensive and spontaneously hypertensive rats. Hypertension. 1979; 1: 378-383.
- Anversa P, Olivetti M, Mellisari M, et al. Stereological measurement of cellular and subcellular hypertrophy and hyperplasia in the papillary muscle of adult rat. J Mol Cell Cardiol. 1980; 12: 781-95.
- Anversa P, Palackal T, Sonnenblick EH, et al. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990; 67: 871-85.
- Olivetti R, Ricci R, Anversa P. Hyperplasia of myocyte nuclei in long-term cardiac hypertrophy in rats. J Clin Invest. 1987; 80: 1818-22.
- Olivetti G, Ricci R, Lagrasta C, et al. Cellular basis of wall remodeling in long-term pressure overload-induced right ventricular hypertrophy in rats. Circ Res. 1988; 63: 648-57.
- Cury DP, Dias FJ, Sosthenes MC, et al. Morphometric, quantitative, and three-dimensional analysis of the heart muscle fibers of old rats: transmission electron microscopy and high-resolution scanning electron microscopy methods. Microsc Res Tech. 2013; 76(2): 184-95.
- Anversa P, Melissari M, Beghi C, et al. Structural compensatory mechanisms in rat heart in early spontaneous hypertension. Am J Physiol. 1984; 246: H739-46.
- Imamura K. Ultrastructural aspect of left ventricular hypertrophy in spontaneously hypertensive rats: A qualitative and quantitative study. Jpn Circ J. 1978; 49: 979-1002.
- Kawamura K, Kashii C, Imamura K. Ultrastructural changes in hypertrophied myocardium of spontaneously hypertensive rats. Jpn Circ J. 1976; 40: 1119-45.
- Wagner C, Ebner B, Tillack D, et al. Cardioprotection by ischemic postconditioning is abrogated in hypertrophied myocardium of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2013; 61(1): 35-41.
- Kotov G, Iliev A, Landzhov B, et al. Postnatal changes in the morphology of the myocardium in rat ventricles. Arch Anat Physiol. 2017; 2(1): 011-7.
- Iliev A, Jelev L, Landzhov B, et al. Neuronal NOS immunoreactivity in the myocardium of the rat during the postnatal period. Compt Rend Acad Bulg Sci. 2016; 69(7): 921-6.
- Iliev A, Jelev L, Landzhov B, et al. Postnatal changes in the myocardium of the rat. A comparative light microscopic and immunohistochemical study. Compt Rend Acad Bulg Sci. 2016; 69(4): 505-12.
- Iliev A, Kotov G, Landzhov B, et al. A comparative analysis of capillary density in the myocardium of normotensive and spontaneously hypertensive rats. Acta Morphol Anthropol. 2017; 24: 19-25.
